Special Topics
Our Special Topics section features items related to current issues, as well as ongoing issues of interest to our stakeholders.
Each topic includes background information as well as links to further information where relevant.
The UK left the European Union on 31 January 2020 on the basis of the Withdrawal Agreement which was agreed by the European Council on 17 October 2019. The agreement includes a transition period until 31 December 2020. The period for the UK government to seek an extension to the transition period has now expired.
Negotiations are ongoing between the EU and the UK on a new future relationship agreement, which if agreed, is due to come into effect from 1 January 2021.
The Health Products Regulatory Authority’s (HPRA) priority, working with all relevant stakeholders, is to ensure continuity in the supply of health products and continued access to products for patients. The guidance provided in this document on the key issues facing the HPRA and our stakeholders is based on the premise that as a result of future relationship negotiations the UK will become a third country at the end of the transition period.
As the UK will become a third country, following Brexit, we would like to draw your attention to the fact that import of tissues into Ireland from outside of the European Union (EU), i.e. a third country, must only take place under an authorisation by the HPRA.
The following guides have been published to provide further information to our stakeholders in relation to Brexit and tissues and cells.
Brexit Guide for Stakeholders - Organisations Responsible for Human Application for Tissues and Cells - Dentists
Brexit Guide for Stakeholders - Organisations Responsible for Human Application of Tissues and Cells - Hospitals
Brexit Guide for Stakeholders - Tissue Establishments
This update includes information on a database of vigilance information collected by the SOHO V&S (Vigilance and Surveillance of Substances of Human Origin) Notify Project in conjunction with the World Health Organisation (WHO). The database allows a user to search over 1800 references describing adverse outcomes in transplantation (organs and tissues and cells) and in assisted reproduction. The user can search by substance type (all organs, one specific organ, all tissues and cells, one specific tissue etc.), incident type (infection, malignancy etc.) or by using key words or phrases.
*Please note the below was originally published prior to our name changing to HPRA in July 2014 and therefore references the name Irish Medicines Board (IMB).
Vigilance and Surveillance of Substances of Human Origin (SOHO V&S project)
This update provides information in relation to further outputs from the SOHO V&S Project (Vigilance and Surveillance of Substances of Human Origin). Two separate clinical user guidelines on Tissues and Haematopoietic Stem Cells have been finalised and are available to view. These guidelines are addressed to healthcare professionals who, working in different organisations responsible for human application (ORHA) in the EU, such as, hospitals, clinics, doctors' surgeries and dentists' surgeries (offices) are involved in processes associated with the management and use of tissues and cells. They aim to define the roles and responsibilities of these healthcare professionals in relation to supporting vigilance and safety of tissues and cells for transplantation.
*Please note the below was originally published prior to our name changing to HPRA in July 2014 and therefore references the name Irish Medicines Board (IMB).
Clinical Users Guides Tissues and Haematopoietic Stem Cells
Commission Directive (EU) 2015/565 amending Directive 2006/86/EC as regards certain technical requirements for the coding of human tissues and cells was published on 9 April 2015.
This Directive gives effect to Article 10 of Directive 2006/86/EC which requires that a Single European Code be allocated to all donated material at a tissue establishment in order to:
- Ensure proper identification of the donor;
- Ensure traceability of all donated material;
- Provide information on the main characteristics and properties of tissues and cells.
Tissue establishments are required under Directive (EU) 2015/565 to affix a Single European Code (SEC) to tissues and cells distributed for clinical application in the EU. The SEC is a unique identifier that consists of two elements; a donation identification sequence, indicating the origin of the tissue or cells, and a product identification sequence, classifying the type of tissue or cells.
The structure of the SEC shall be as follows:
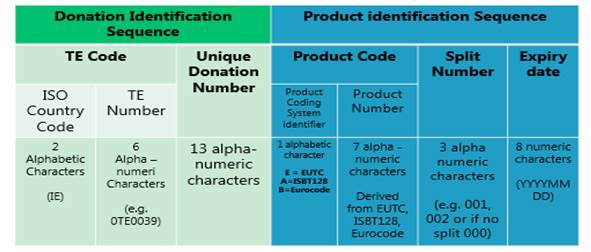
An EU Coding Platform will be established and hosted by the European Commission which will contain a register of authorised EU Tissue establishments (the EU Tissue Establishment Compendium) and a register of all tissues and cells circulating in the Union (the EU Product Compendium). The Product Compendium will facilitate the application of the relevant product code under the three permitted coding systems (EUTC Code, Eurocode and ISBT 128). A translator application will be developed that will allow searches of both compendia in order to construct the SEC.
Member States are required to transpose the provisions of Directive (EU) 2015/565 into their national legislation by 29 October 2016 and the requirements will apply from 29 April 2017.
In preparation, we request that you review the Directive and circulate to relevant personnel at your establishment. We advise Tissue Establishments to start planning for the implementation of the Directive now. The HPRA will be available to discuss the requirements and aid in the transition.
Supporting Information:
In 2013 and 2015, the HPRA hosted two information sessions to highlight the requirements of this Directive. The presentations provided at the information session held in 2015 are available here;Presentation to Tissue Establishments / Presentation to ART Tissue Establishments.
In addition, the European Commission has issued an information document and question and answer document which contains further useful information.
For Tissue Establishments who intend to incorporate ISBT 128 labelling as part of the SEC, further information can also be found on the ICCBBA website: ISBT 128 and the Single European Code (SEC).
The new EU Coding platform for tissues and cells is officially launched on the 6th of October 2016. The link to the EU Coding Platform is available here: https://webgate.ec.europa.eu/eucoding/.
The EU Coding platform will allow public, free of charge access to the EU Tissue Establishments Compendium and the EU Tissue and Cell Product Compendium. The EU Coding Platform also includes a translator application that will allow searches of both compendia in order to construct the Single European Code.
Commission Directive 2015/566 implementing Directive 2004/23/EC as regards the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells was published on 8 April 2015.
Directive 2004/23/EC requires that Member States and importing tissue establishments ensure that imports of tissues and cells meet equivalent quality and safety standards to the ones laid down in the directive and calls for the establishment of procedures to verify the equivalency of the quality and safety standards of imports of tissues and cells.
Directive 2015/566 lays down these procedures for verifying the equivalency of the quality and safety of imports of tissues and cells and includes procedures to be followed by importing tissue establishments in their relations with third country suppliers.
Member States are required to transpose the provisions of Directive (EU) 2015/566 into their national legislation by 29 October 2016. Application of the requirements is required from 29 April 2017.
In preparation, the HPRA requests that the Directives are reviewed and circulated to all relevant personnel at Tissue Establishments. We advise Tissue Establishments to start planning for the implementation of this Directive. The HPRA will be available to discuss the requirements and aid in the transition as required.
Supporting Information:
In October and November 2016 the HPRA provided refresher training and updates on the Single European Code and Import Directives to Tissue Establishments. The presentations provided are available here:
Presentation to Tissue Establishments
Presentation to ART Tissue Establishments
In 2015, the HPRA hosted two information sessions to ART and non-ART Tissue Establishments to highlight the requirements of this Directive.
The presentations provided at the information session held in 2015 are available here;
Presentation to Tissue Establishments
Presentation to ART Tissue Establishments