Automated external defibrillators
An automated external defibrillator (AED) is a medical device that analyses a person’s heart rhythm and, when needed, delivers a shock to sudden cardiac arrest (SCA) victims who are in a shockable heart rhythm.
A defibrillator can play a potentially lifesaving role. Used correctly, it can improve a person’s survival chances following SCA. Therefore, defibrillators need to be accessible and in good working order at all times in the event that they are needed for an emergency situation.
Traditionally AEDs have been operated by trained healthcare professionals such as GPs as well as those working in the ambulance and fire brigade services. As awareness of their benefits has grown, they are now widely used in community locations, such as sports facilities and shopping centres and often operated by members of the public.
This webpage provides advice on selecting and purchasing an AED for use in a community setting. It also gives recommendations for maintaining the device after it has been purchased.
*Please note where used on this page the term ‘defibrillator’ is specifically referring to the AED family of defibrillators and does not refer to other types of defibrillators such as implantable cardiac defibrillators.
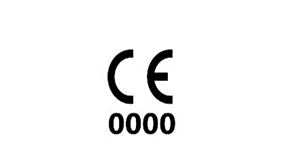
On a defibrillator, there should be a four digit number below the CE mark.
Other useful information
This webpage highlights some of the important issues that should be considered when purchasing, storing and maintaining an AED. It is not intended to be a guide on how to use a defibrillator nor does it replace or reduce the importance of your defibrillator manual and training.
The following sources of information may also be of interest:
• Irish Heart Foundation
• National Ambulance Service
• Pre-Hospital Emergency Care Council
Relevant training courses may be found through the websites listed above.
How to report an incident to the HPRA
If your defibrillator poses a risk to the health and safety of the patient, please report the problem to the manufacturer and to the HPRA. You should report any unexpected problem or malfunction that may affect the patient’s health or cause or contribute to an injury. For example, failure of the status indicator on the device to alert you that the device is not operating when in use.
You can report incidents to the HPRA by filling in our online user report form. If you would prefer to fill out a printed copy of the form, you can download it from our website or request a copy by phone or email.
Print/PDF versions
This webpage is also available as a leaflet in PDF or print format. You can request a copy by emailing communications@hpra.ie.