HPRA guide to conducting clinical trials under the CTR in Ireland
Key features of the Clinical Trials Regulation
The key features of the new Clinical Trial Regulation include:
-
Identical rules for conducting clinical trials throughout the European Union (EU);
-
Increase in the efficiency in approval process for clinical trials;
-
Single submission and approval of multinational clinical trial applications through an EU ‘Clinical Trial portal and database’ (known as the Clinical Trial Information System (CTIS);
-
A harmonised procedure for assessment by member states, divided in two parts;
-
Strictly defined deadlines for assessment;
-
Involvement of the ethics committees in the assessment procedure.
The above will assist the Clinical Trial Regulation in achieving its aim of creating a favourable environment for conducting trials in the EU while also ensuring that the highest standards of safety for participants are maintained. The Clinical Trial Regulation also aims to increase the transparency of clinical trial information with details on the authorisation and results of EU trials among the information to be made publicly available.
Timeline and transition to the Regulation
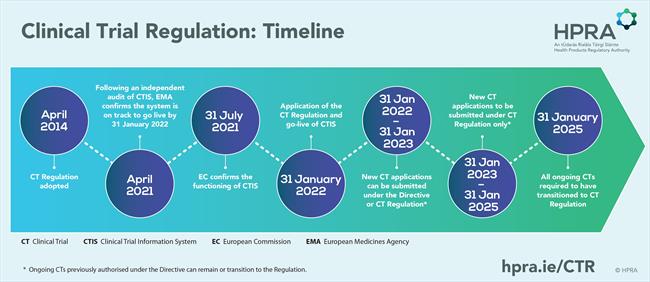
Please click on the image to view in full size.
On 31 July 2021, the European Commission published its decision, via the Official Journal of the European Union, that the EU portal and EU database had achieved full functionality. The Clinical Trial Regulation (Regulation (EU) No 536/2014, and the supporting Clinical Trial Information System (CTIS) came into effect on 31 January 2022. A three-year transition period now applies for transition from the Clinical Trials Directive (Council Directive 2001/20/EC; CTD) to the CTR.
The transition period sets out the following key timelines:
-
From 31 January 2022 until 31 January 2023 – All initial clinical trial applications can be submitted under either the Directive or the Clinical Trial Regulation.
-
From 31 January 2023 – All initial clinical trial applications need to be submitted under the Clinical Trial Regulation.
-
From 31 January 2022 until the end of the transition period – Ongoing clinical trials previously authorised under the Directive can remain under the Directive, or they can transition to the Clinical Trial Regulation.
-
By 31 January 2025 – All ongoing clinical trials will be required to have transitioned to the Clinical Trial Regulation and will need to be migrated to CTIS.
Additional information in relation to the transition period and eligibility of clinical trials can be found on the Heads of Medicines Agency (HMA), Clinical Trials Facilitation and coordination Group (CTFG) Best Practice Guide for sponsors of multinational clinical trials.
Further guidance and documents
The HPRA has published its guide to clinical trials conducted under the CTR in Ireland. Additionally, the following guidance and documents may be of assistance to stakeholders in further understanding the implementation of the Clinical Trial Regulation:
-
The European Medicines Agency has released a Clinical Trial Information System (CTIS) – Sponsor Handbook which aims to provide clinical trial sponsors with the information required for using CTIS. The handbook will be updated as more information becomes available and sponsors are encouraged to check that they are referencing the most up-to-date version at the time. The EMA also welcomes feedback on the CTIS Sponsor Handbook and any proposals or comments can be submitted directly to the EMA via this feedback form.
-
The European Commission has released a set of documents that will be applicable to clinical trials under the Clinical Trial Regulation.
-
The European Commission has published a Question and Answer document on the 'EudraLex - Volume 10 - Clinical trials guidelines' webpage under 'Chapter V - Additional documents'.
-
EC-DG Sante/HMA-CTFG/EMA joint training on the Clinical Trials Regulation (EU) 536/2014: On 9 and 10 March 2021, to support Member States (MSs) in preparation for the launch of the Clinical Trial Regulation DG Sante of the European Commission, the European Medicines Agency and the Clinical Trials Facilitation and coordination Group of Heads of Medicines Agency organised a two day training event. Members of the competent authorities and the ethics committees from the various MSs attended. The two day training aimed to provide an overview of key changes proposed under the incoming Clinical Trial Regulation and included presentations on a number of different topics.
-
Guidance and data fields required to be completed by sponsors in the submission and management of a clinical trial in CTIS are available on the EMA website.
-
EudraVigilance training is being provided to clinical trial sponsors by the EMA from March 2022. The training will provide information to sponsors who will directly report suspected unexpected serious adverse reactions (SUSARs) to EudraVigilance via EVWEB. More information can be found on the EMA EudraVigilance training and support page.
Clinical Trials Information System (CTIS)
The introduction of the Clinical Trial Regulation has also seen the introduction of the new Clinical Trial Information System (CTIS). This will act as a single entry point for submitting clinical trial information in the European Union (EU) and will support the daily business processes of Member States and sponsors throughout the life cycle of a trial. Additionally, CTIS will also assist in making clinical trial information in Europe more accessible and transparent.
The EMA has provided an infographic with links to key information for sponsors on CTIS including training and support, the CTIS sponsor handbook, CTIS newsletters, and information on the Clinical Trials Regulation (CTR).
What can sponsors do now?
The HPRA has the following advice:
-
Sponsors are encouraged to review their current clinical trial portfolios and identify the trials that they plan to submit under the Regulation.
-
Sponsors should review their current portfolios to ensure that there are no outstanding submissions relating to the life cycle of any of their trials e.g. end of trial declarations or end of trial summary report.
-
Sponsors should give consideration to how they plan to interact and manage their trials under the CTR and the associated CTIS as both mark a significant departure from current processes.
-
It is strongly recommended that both commercial and non-commercial/academic sponsors review the extensive and comprehensive training materials that are available from the EMA website and monitor both the EMA and HPRA websites for further updates. The EMA has also provided an infographic with key information for sponsors on CTIS.
-
Sponsors are advised to review the presentations from the HPRA's Clinical Regulation Information Week, run in conjunction with the National Office for Research Ethics Committees and the Department of Health, held on the week of 22 November 2021. Recordings and PDF versions of the presentations can be located under the webinar tab located on this webpage.
-
CTIS interacts with various EMA existing databases and systems such as IAM (register users), OMS* (search for organisations) and xEVMPD (search medicinal products). Sponsors must ensure that the relevant data is registered in these databases prior to submitting an initial clinical trial application. Sponsors who are unsure of their OMS registration status can review this on the EMA’s Substance, product, organisation and referential portal. Sponsors wishing to know more about OMS registration, including site registration, should consult both the Clinical Trials Information System (CTIS) - Sponsor Handbook and the CTIS highlights newsletter.
-
An EMA account is required to access the CTIS restricted workspace and users of other EMA applications (e.g. EudraVigilance) can use these log in details to access this. Information specific to registering a Sponsor administrator can be located in the EMA CTIS online modular training programme with particular reference to module 3 and 19 and the accompanying step-by-step guides and video tutorials.
-
When considering user registration in CTIS organisations should also give some thought as to whether they wish to assume an Organisation-Centric or CT-Centric approach and more information regarding this can be located in module 7 of the EMA CTIS online modular training programme.
*Additional OMS information
The EMA have set up specific trouble shooting sessions to allow stakeholders the opportunity to address issues and questions in relation to OMS. The events will run throughout 2022 and stakeholders wishing to know more can visit the EMA events page.
Organisation data can be retrieved in CTIS after the go-live. It is highly encouraged for organisations to register in OMS before using the CTIS to facilitate the process.
In OMS, an organisation should have one ORG ID, and under that ORG ID they enter the same amount the corresponding of location entries (each with the respective LOC ID) as they have locations/physical address; e.g. one hospital (1 ORG ID) with two physical addresses, and therefore two locations, has 2 LOC IDs under the same organisation ORG ID.
However, please note that details of the departments (acting as trial sites) of the hospitals are not to be registered in OMS and they will be collected, locally, only in CTIS at the time of submission of the CTA. This would appear like this in CTIS:
The EMA has published two new documents in order to help future users get prepared for CTIS regarding OMS registration:
Should you have any more OMS-related queries, please contact OMS Service Desk as instructed in pages 44-45 of the ‘OMS web user manual’, found on the OMS website by clicking the ‘Help’ tab.
Registration of Processes Exempted under Article 61(5) of the Regulation
Article 61(5) of the Clinical Trial Regulation (CTR) (EU Regulation 536/2014) provides an exemption from the requirement to hold a Manufacturer’s Authorisation for some processes relating to investigational medicinal products (IMP).
The exemption applies to processes which are carried out by authorised staff at a hospital, health centre or a clinic participating in the clinical trial.
Under Article 61(5) of the CTR, exemptions for the following processes apply:
- re-labelling or re-packaging of the IMP
- preparation of radiopharmaceuticals used as diagnostic IMPs
- preparation of an IMP in accordance with a doctor’s prescription or in accordance with a pharmacopeial monograph
The European Union (Clinical Trials on Medicinal Products for Human Use) (Principal) Regulations 2022 defines, at national level, the persons authorised to carry out these activities and includes a retail pharmacy business within the definition of a clinic.
These national regulations also describe the requirement for the HPRA to maintain a ‘Register of Exemptions’ in relation to processes described above. As of 31 January 2023, where the above processes are carried out with respect to a clinical trial falling under the CTR, the process must be included on the Register of Exemptions maintained by the HPRA.
Applications for inclusion of a process on the Register of Exemptions should be made on the Application for Register for Exemption form. The application form for registration and further guidance on the Register of Exemptions and the appropriate requirements which apply for these processes are available on the HPRA website.
The Minister for Health may publish further information on the appropriate requirements to be applied for those processes conducted Article 61(5) of the CTR.
SME and academia
A specific Clinical Trials Information System (CTIS) training module for SMEs and Academia has been produced by the EMA and can be found on the CTIS online modular training programme page of the EMA website under module 19. SME and Academia are strongly encouraged to consult the module which covers important aspects such as user access management and how to create, submit and withdraw a clinical trial application.
Webinar for small and medium-sized enterprises (SMEs) and academia on the Clinical Trials Regulation and the Clinical Trials Information System (CTIS)
An EMA webinar for small and medium-sized enterprises (SMEs) and academia on the Clinical Trials Regulation (CTR) and the CTIS was held on 29 November 2021. The webinar included an overview of the CTR, an introduction to the new process for submitting clinical trials as well as the functionality of CTIS. More information regarding the webinar, including a recording of the event, can be located on the event information page.
Dedicated training webinars detailing essential information on CTIS for micro, small and medium-sized enterprises (SMEs) and non-commercial (academic) sponsors
The first training session, held on 22 February 2021, provided an overview of CTIS, user access management (including how to register users), sponsor user management and sponsor roles and permissions in CTIS. Further information can be found on the EMA's webpage for Day 1 of the webinar.
The second training session, held on 4 March 2021, covered submitting an initial trial application in CTIS, updating an initial trial application and making substantial modifications along with adding a concerned Member State, making non-substantial modifications and submitting trial results. For more information please see the EMA's webpage for Day 2 of the webinar.
Webinars – November 2021
The new Clinical Trials Regulation explained
In advance of the Clinical Trial Regulation (Regulation No 536/2014) coming into effect on 31 January 2022, the HPRA, in conjunction with the National Office for Research Ethics Committees, held a series of one–hour webinars from Monday 22 to Thursday 25 November to help stakeholders understand the new requirements.
The webinars covered topics such as an overview of the Regulation, including post-authorisation and compliance aspects, and guidance on next steps for sponsors and investigators. The Department of Health provided an update on the national framework for the Regulation.
The aim of the webinars was to present the main changes that those involved in clinical trials in Ireland can expect when the Regulation is implemented, and throughout the three-year transition period that will follow.
Note on website cookies
Please note that the videos are only available to view if you have selected the relevant preferences in the cookie settings for our website. The videos are also available to view on the HPRA YouTube account.
Monday 22 November: General principles and new concepts
The following presentations are included in the video below:
These presentations can be downloaded by clicking on the titles above.
Tuesday 23 November: Post-authorisation, transition and how can I prepare?
The following presentations are included in the video below:
These presentations can be downloaded by clicking on the titles above.
Wednesday 24 November: Ireland - how will we implement?
The following presentations are included in the video below:
These presentations can be downloaded by clicking on the titles above.
Thursday 25 November: Ireland - Compliance aspects
The following presentations are included in the video below:
-
Serious breaches – Norah Cassidy, GCP/PV Inspector, HPRA
-
GCP inspections - changes brought about by the CTR - Peter Twomey, GCP-PV Inspection Manager, HPRA
-
IMP manufacture and labelling - Paul Sexton, GMP Policy Manager, HPRA and Peter Twomey, GCP-PV Inspection Manager, HPRA
-
Close of event - Grainne Power, Director of Human Products Authorisation and Registration, HPRA
These presentations can be downloaded by clicking on the titles above.
More references and information sources on the new Clinical Trial Regulation can be found here.